Apple AirPods Pro 2 Gets FDA Nod For Game-Changing Hearing Aid Feature: Here’s Why It Matters
The Food and Drugs Administration (FDA) of the US has approved the new hearing aid feature onboard Apple’s AirPods Pro 2, making it the country’s first over-the-counter hearing aid software device. This has cleared the way for the company to roll the feature out in the near future.
During the recent iPhone 16 launch event, Apple updated its AirPods lineup with two models of the AirPods 4. And though no successor to the AirPods Pro 2 was announced, the hearing aid update became the big talking point for owners and potential buyers of the high-end earbuds.
The FDA has now fully authorised the use of the hearing aid feature on the second-gen AirPods Pro. This comes after tests with 118 individuals were conducted and the results showed that subjects using the functionality “achieved similar perceived benefit” to those who were wearing professional hearing gadgets.
According to the FDA, these tests even showed that the levels of sound amplification in the ear canal and speech understanding in noise were pretty much similar to those of the hearing aid equipment.
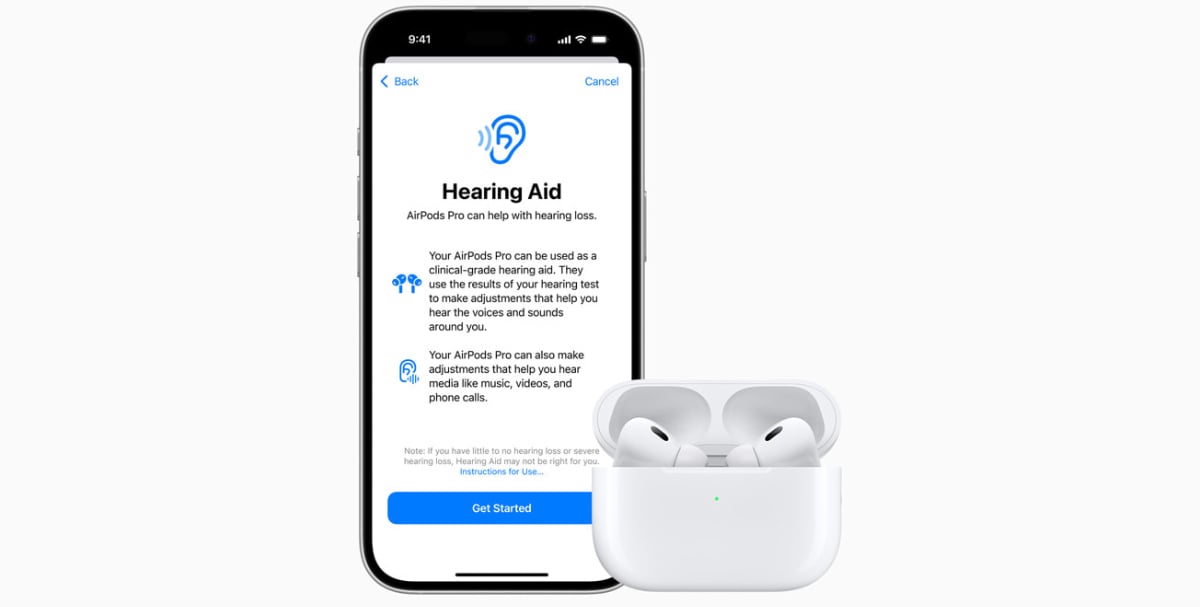
During the Apple event, the company gave a detailed explanation of how the AirPods Pro 2 would function as hearing aids. Users will be asked to take a hearing test that will involve them tapping their smartphone screens on hearing certain sounds.
These responses will then be used to tune the hearing aid feature and create personalized audio profiles tailored to their requirements. This update with the hearing aid functionality will be released for AirPods Pro 2 users in more than 100 countries and regions this fall.
The AirPods Pro 2, which come with Apple’s famed ANC feature, are officially priced at $249 but can be snagged for much lower values via most retailers.
Was this article helpful?
YesNo